PLASIMO's automatic reduction tool uses the Pathway Analysis (PA) method to reduce the chemistry in a Global Model. The resulting reduced chemistry provides insight into the important species and reactions in a model. Such reduced chemistry can be more practically used in spatially resolved models.
The PA tool analyses the results of the GM containing the complete chemistry and suggests a reduced chemistry based on a certain rate threshold. For time-varying plasmas this analysis can be made at different moments in time. Then the GM is run with the suggested reduced chemistry. The rate threshold is varied and the PA and GM are consecutively run until the user-specified tolerance (deviation from the complete model results) is reached.
An output of the automatic reduction tool is a “blacklist” containing the species and reactions that can be removed from the chemistry. Such list can be conveniently used in further simulations that used this kind of chemistry.
Examples of model reduction
Example: H2 chemistry
A global hydrogen model includes 40 species participating in 362 reactions. The pressure is set to 5 Pa and the gas temperature is kept constant at 300 K. The input power density is shown in figure 1.
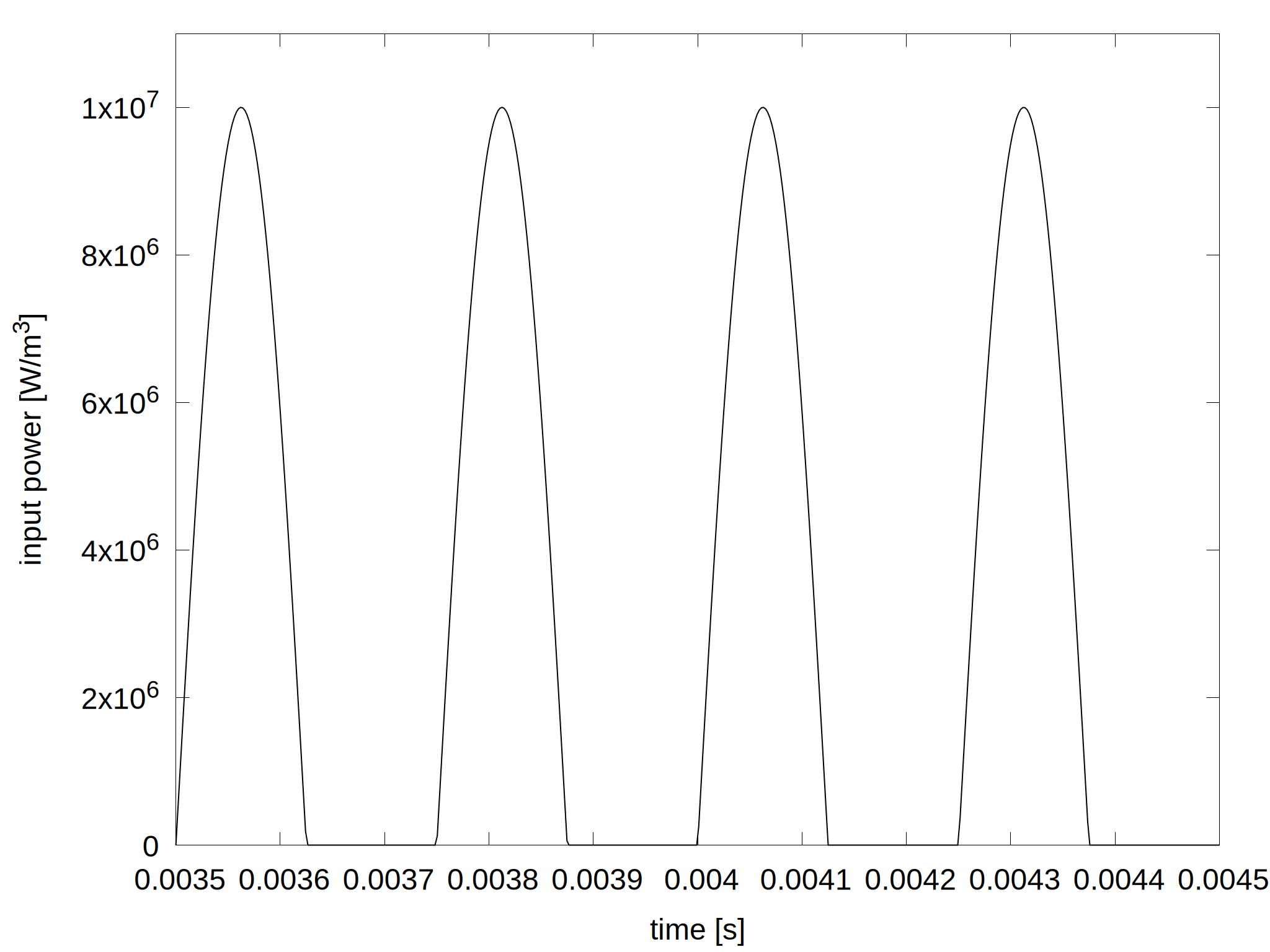
Figure 1: Applied power density as a function of time.
PLASIMO's automatic reduction tool is used to reduce the number of species and reactions for this model. Two reductions are done:
- Reduced model with a maximum relative tolerance of 0.4% results in a model with 24 species and 61 reactions
- Reduced model with a maximum relative tolerance of 7% results in a model with 21 species and 56 reactions
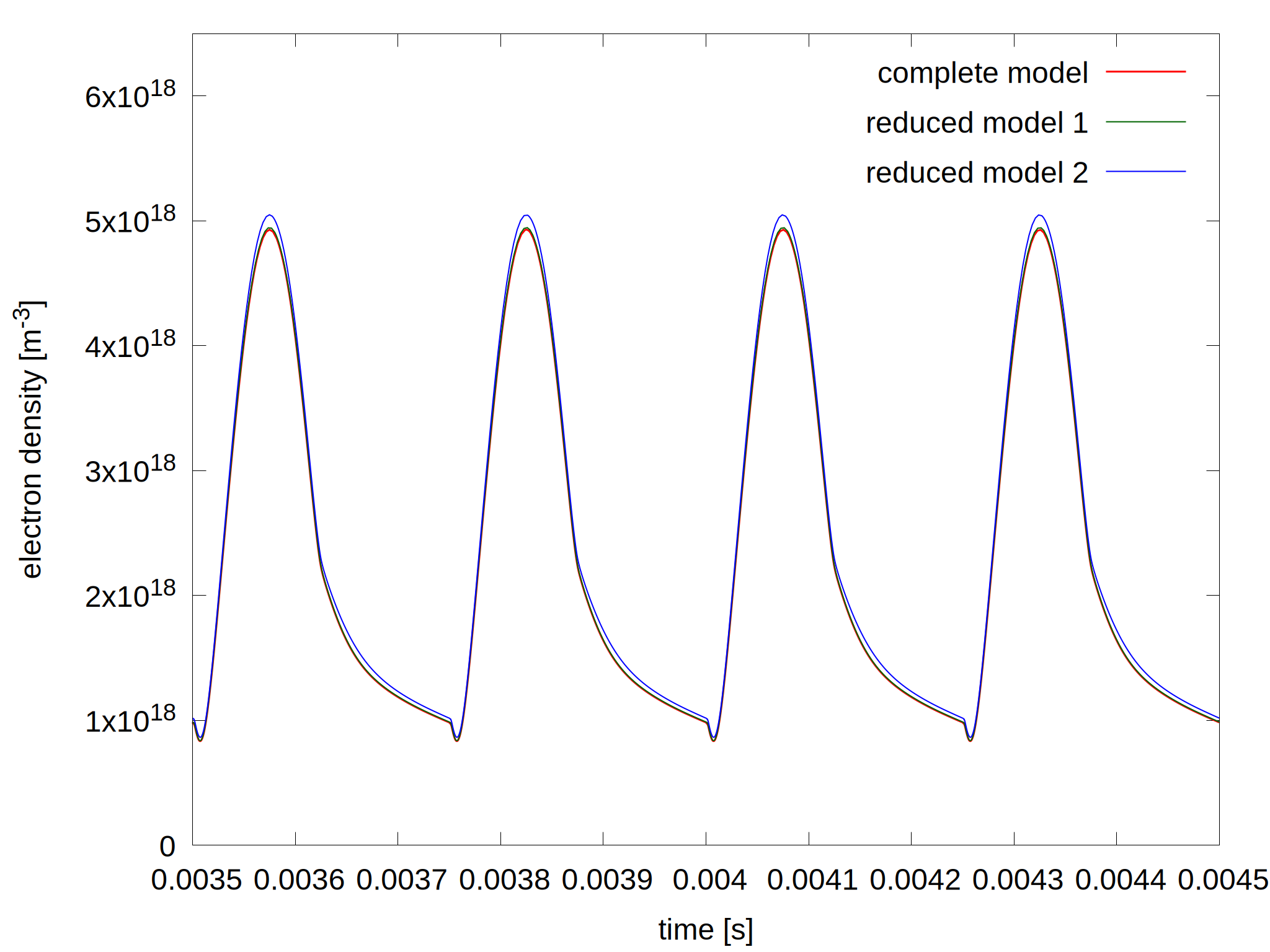
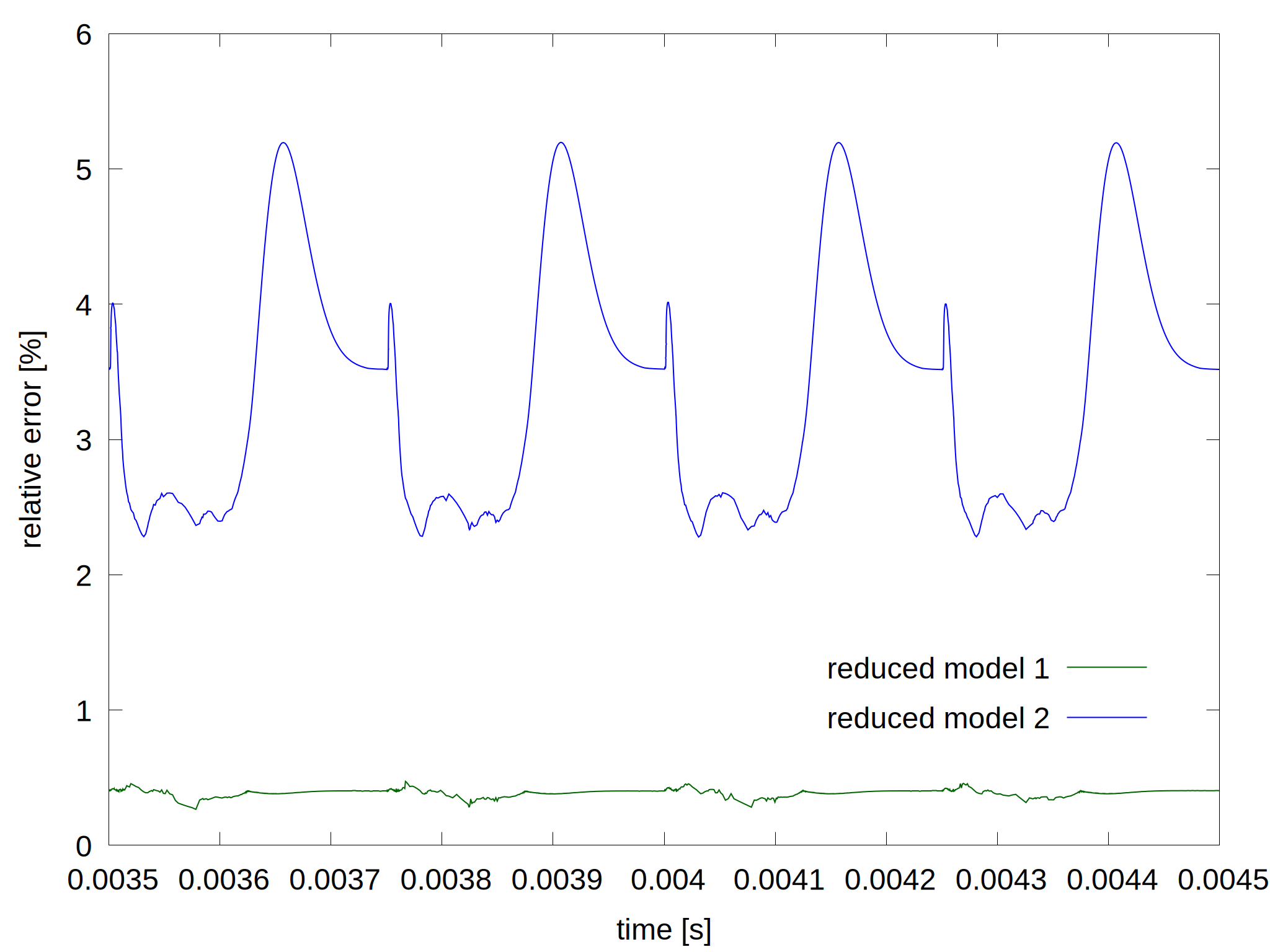
Figure 2: Plasma density as a function of time for the complete model and the reduced models (left) and the relative error for the two reduced model (right). The last 4 pulses of the simulations are shown where quasi steady state is established.
As more species and reactions are removed, the deviation from the complete model gets larger. For this particular case, PLASIMO's reduction tool determined that about half of the species and 85% of the reaction can be removed, while the results will deviate from the original model with less than 7%. At the first reduction, vibrational states higher than 3 are removed, 3 electronically excited sates and H-. For the second reduction, 3 more electronically excited states are removed.
The reduction of such chemistry takes about a couple of minutes as most of the time is spent on running of the complete model. The result is a comprehensive reaction pathway analysis, insight into the important pathways in the chemistry and a reduced model for the particular conditions without any human intervention.
Example: H2O-He chemistry
The H2O-He plasma model is the same as described in Tadayon et al [1]. The model is freely available and can be downloaded in PLASIMO format from Resources.
PLASIMO's automatic chemistry reduction tool was used to reduce the chemistry of the H2O-He microwave model at different values of the input power. In general, a reduced model at a given operating parameters may not be applicable to the model at other operating conditions. To that end, the automatic reduction was applied to the models at different power separately. For all models, three reductions are done where a maximum tolerance of 1%, 5% and 10% is set only for the electron density and electron temperature.
Figures 1 and 2 show results from the complete model and the three reduced models together with their relative errors. As expected, the relative errors are within the requested tolerance.
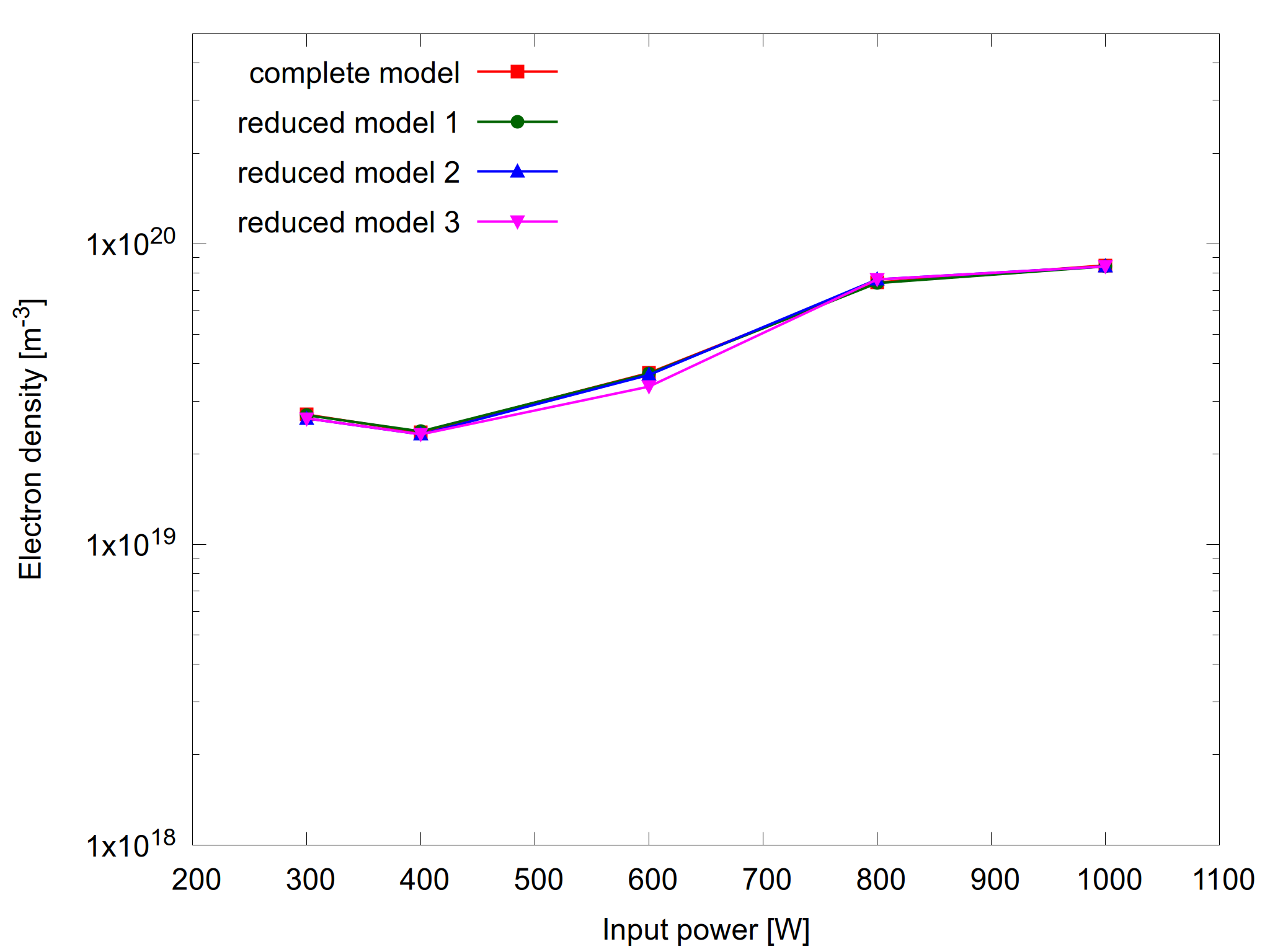
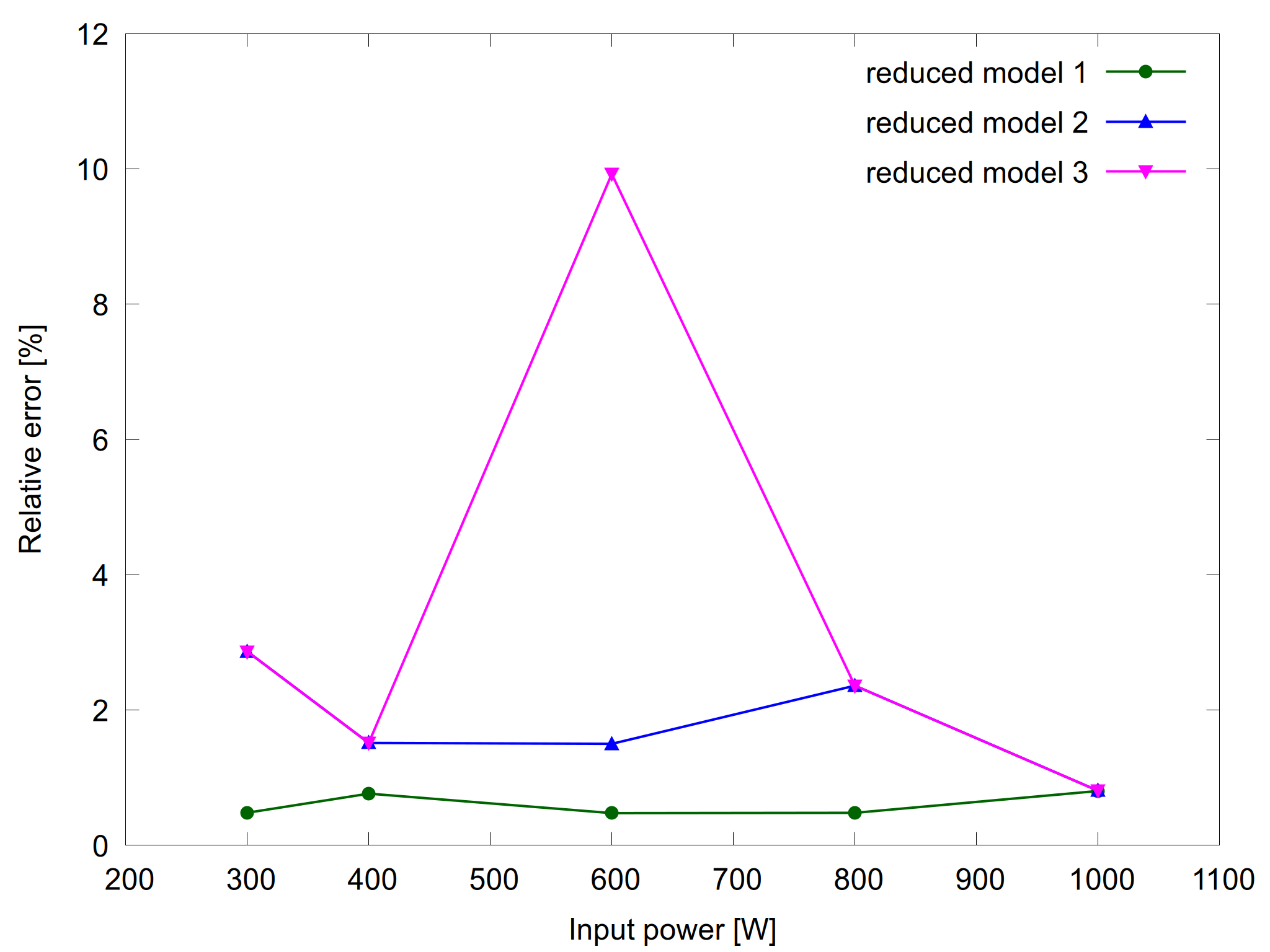
Figure 1: Plasma density as a function of input power for the complete model and the reduced models (left) and the relative error for the reduced models (right).
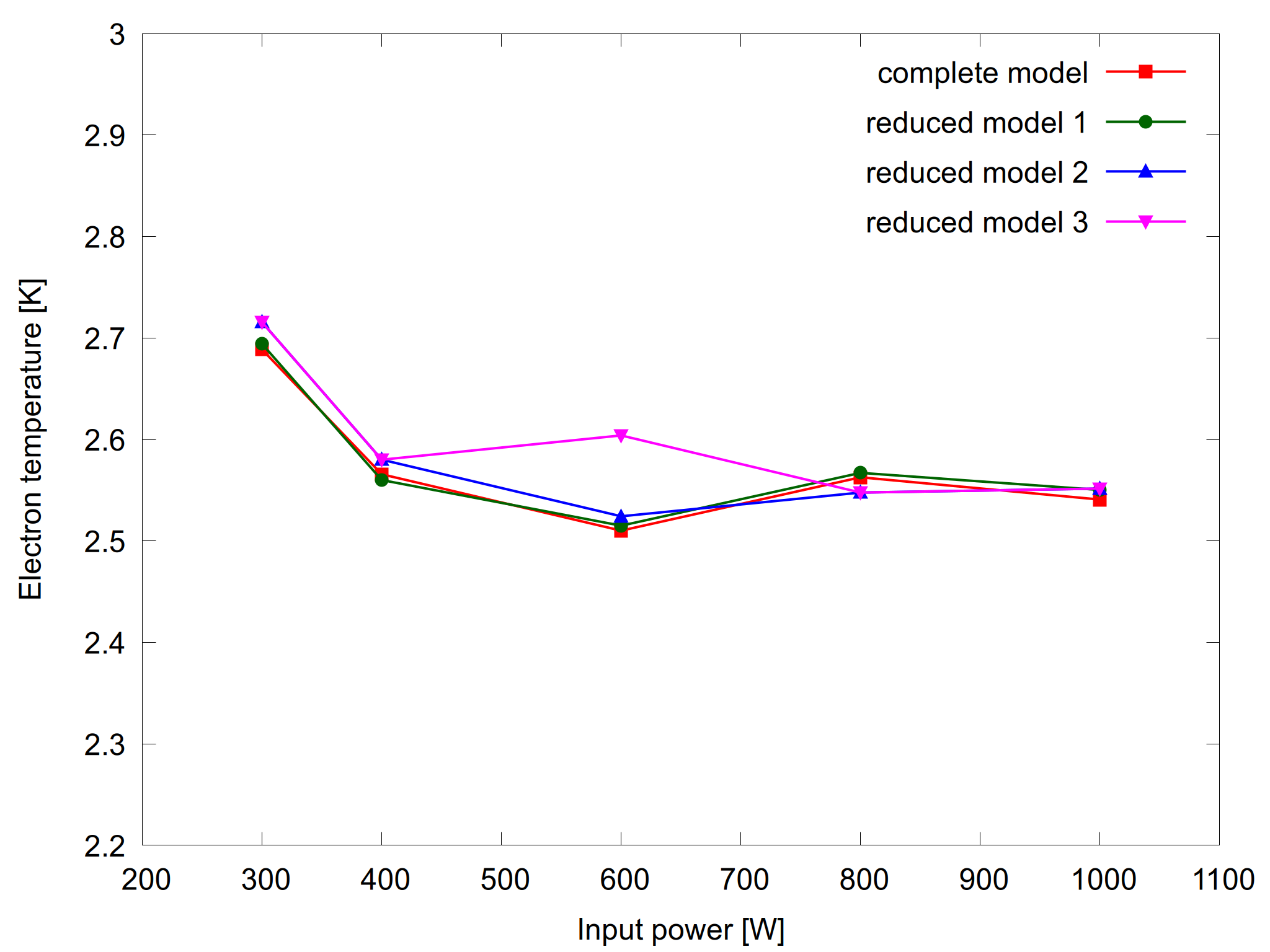
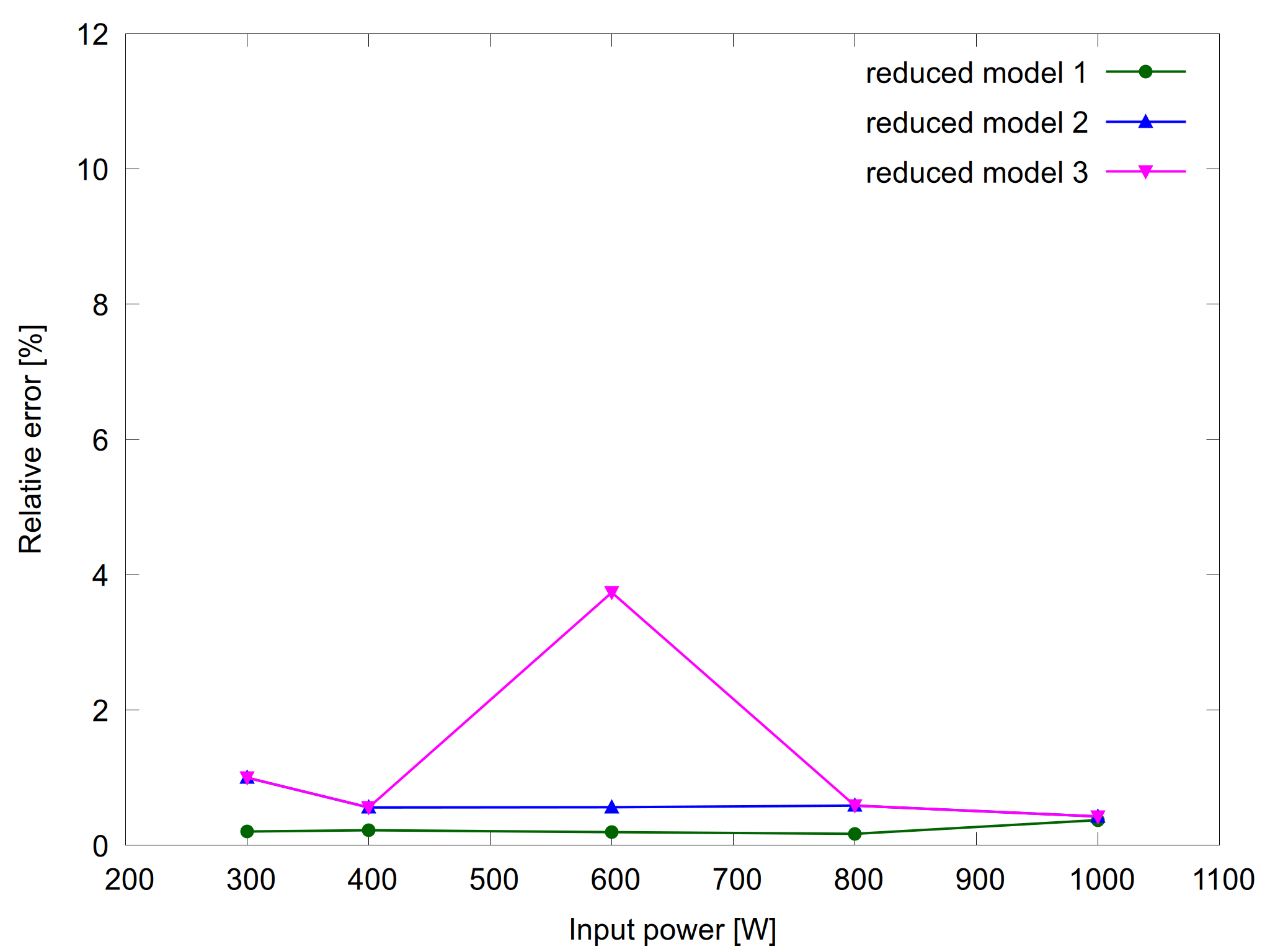
Figure 2: Electron temperature as a function of input power for the complete model and the reduced models (left) and the relative error for the reduced models (right).
Note that no constraints were specified for the other species. That means that their relative error may exceed the requested tolerance (for the electron density and electron temperature). For example for H3O+ the relative error can reach values up to 30%, as shown on figure 3. For other species like H2O2 the reduced models show less that 2% deviation from the complete model. This is also the case for many other variables, such as atomic oxygen density and gas temperature.
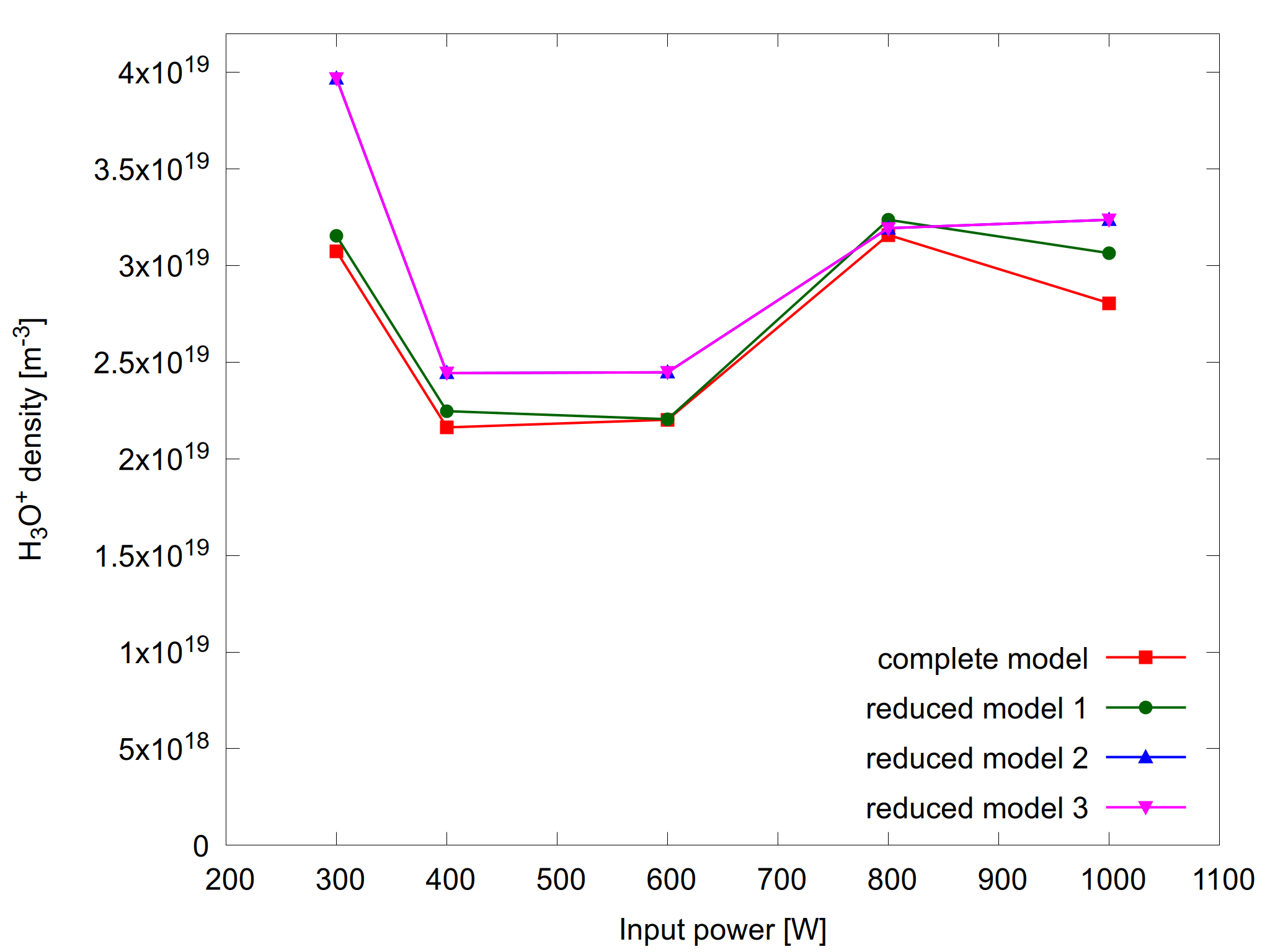
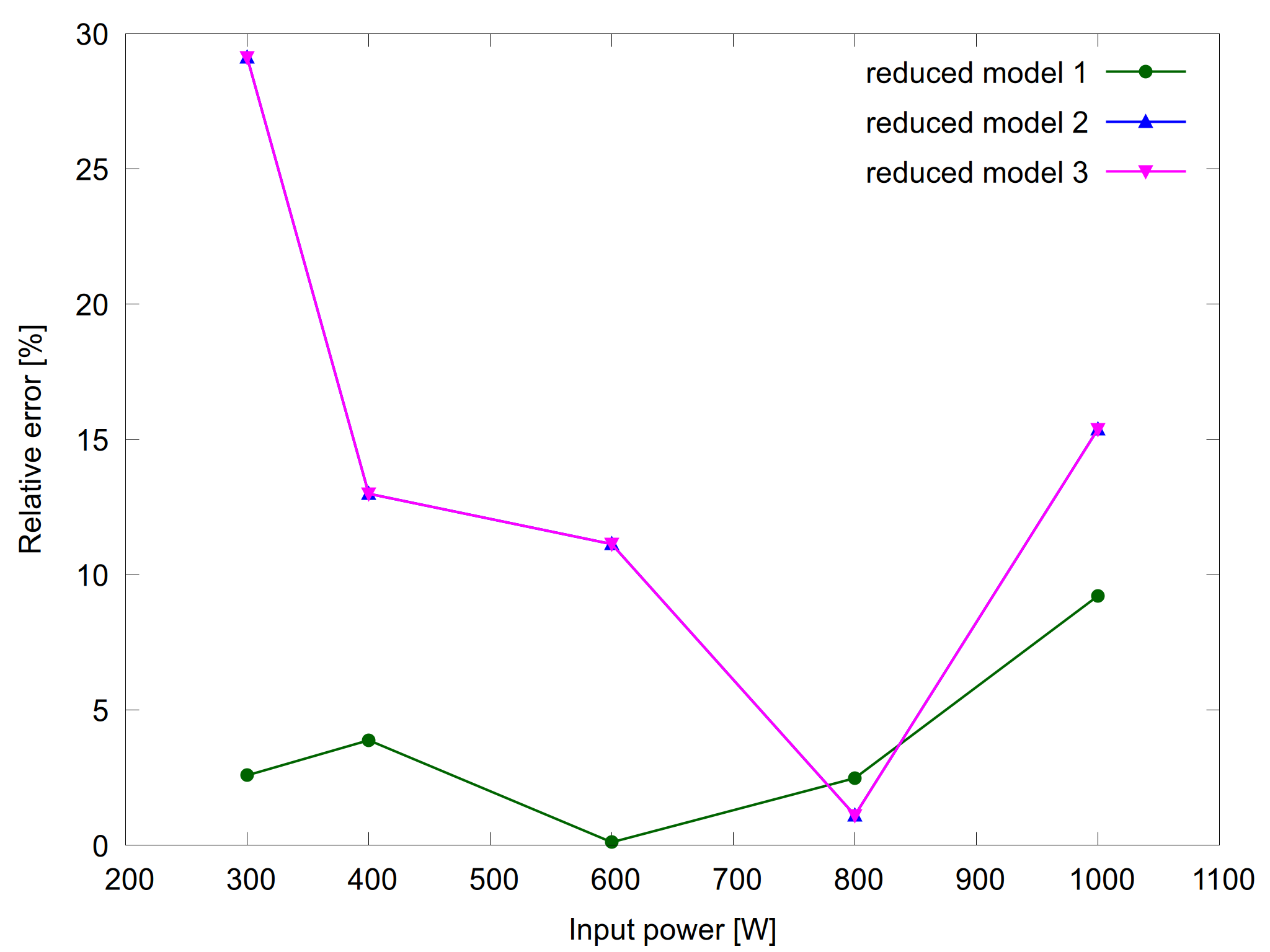
Figure 3: H3O+ density as a function of input power for the complete model and the reduced models (left) and the relative error for the reduced models (right).


Figure 4: H2O2 density as a function of input power for the complete model and the reduced models (left) and the relative error for the reduced models (right).
The number of reactions and species that can be removed in a chemistry is strongly dependent on the number of constraints and relative error that are specified at specific operating conditions. For this reason a general reduction is not possible and the required chemical accuracy should be considered carefully.
References
[1] S. Tadayon Mousavi, E. A. D. Carbone, A. J. Wolf, W. A. Bongers and J. van Dijk (2021). Two-temperature balance equations implementation, numerical validation and application to H2O-He microwave induced plasmas Plasma Sources Sci. Technol. 30, 075007. [ read article ]